Saudi Food & Drug Authority - Medical Devices Marketing Authorisation System
Technologies: IIS, .NET, ASP.NET
Customer Profile
The Saudi Food and Drug Authority, which has a Board of Directors chaired by His Royal Highness Prince Sultan bin Abdulaziz, The Crown Prince, Minister of Defence and Aviation, and The Inspector General, was established under the Council of Ministers resolution No. (1) issued on 10/3/2003, as an independent Authority reporting to the Council of Ministers. The SFDA aims to "ensure the safety of food, safety, quality and effectiveness of drugs, and the safety, quality, efficacy and performance of medical devices according to their intended purpose. Regulating medical devices, in vitro-diagnostic devices, prescription eye glasses, contact lenses and their solutions, are among the responsibilities of SFDA in accordance with its law issued by the royal decree No.(M/6) issued on 13/2/2007.
Business Situation
In accordance with the Food and Drug Authority (SFDA) law issued by the royal decree (M/6) on the 25/ 01/1428 which gave the Saudi Food and Drug Authority the responsibility to regulate medical devices in Saudi Arabia. The council of ministers decision No.181 issued on 3/6/1428 came to stress the importance of regulating medical devices which relate directly to human health. In the last few years, the Saudi health services have grown tremendously. Recognising this, the SFDA required systems to support the new function of regulating medical devices within the Kingdom by issuing marketing authorisation licences to approved establishments.
Solution
Engine Solutions facilitated user requirements workshops where detailed user requirements were discussed and documented. Key workflows were identified which formed the basis of the new system.
Engine Solutions worked with the SFDA to:
- Analyse and document business requirements
- Define and document system requirements specification/functional specification
- Create an implementation plan
- Provide quality assurance of associated implementation project
Results
After this successful engagement the SFDA are now successfully processing marketing authorisation requests from qualifying organisations, as per the requirements of the royal decree.
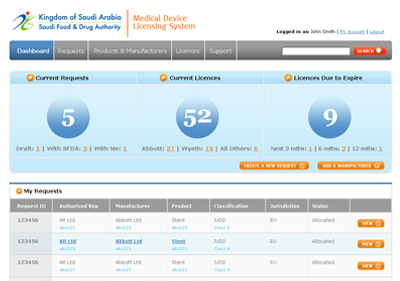
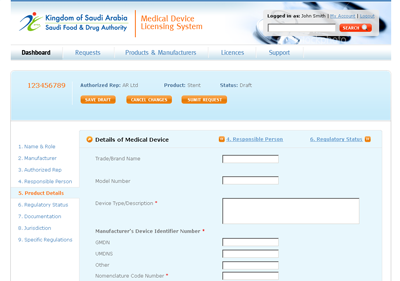
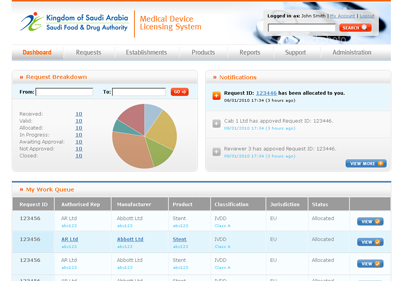
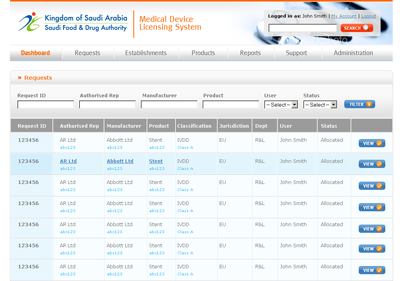
The MDMA system, as designed, is accessible by SFDA staff, CAB users and manufacturers and authorised representatives, with appropriate security controls in place. KPI dashboards provide summary information to authorised users.
Testimonial
"Engine’s role in our Medical Devices Marketing Authorisation project was pivotal to its success; their total commitment to the quality of the deliverables in this high profile project was crucial to the SFDA, and the project’s success could not have been achieved without their world class professionalism and customer service.
Engine Solutions’ abilities and commitment to the SFDA continually instils an unparalleled level of confidence that they will get the job done within the timeframe and on budget, and this commitment has always made Engine stand out."
Meshal Al-Amri | Executive Director, Premarket Approval & Scientific Evaluation Management
Saudi Food & Drug Authority
www.sfda.gov.sa